Olympus Endoscopes Now Powered by Next-Generation Ultrasound Processor
ProSound F75 Ultrasound Imaging Platform enables More Accurate Diagnosis of Cancers in GI Tract

CENTER VALLEY, Pa., May 14, 2013 - Olympus, a precision technology leader in designing and delivering innovative solutions in Medical and Surgical Products, among other core businesses, announced today that the Company’s ultrasound endoscopes will now be powered by the ProSound F75 Ultrasound Processor.
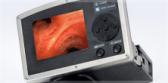
The ProSound F75 Ultrasound Processor, developed in partnership by Hitachi Aloka Medical, Ltd., provides physicians with the highest quality imaging—that aids in more accurate diagnosis of diseases of the GI track and surrounding organs such as the pancreas, bile duct, liver, spleen and gallbladder as well as assessing a variety of cancers. The newly introduced platform improves workflow from pre-examination to after examination for better data management, and provides improved visualization for more accurate blood flow information.
“The ProSound F75 delivers outstanding image quality that facilitates easy identification and more accurate staging of GI tumors in my practice,” said Dr. Shyam Varadarajulu, Medical Director of the Center for Interventional Endoscopy (CIE) and Professor of Medicine at the University of Central Florida. “The images are crisp with excellent resolution and the technology is truly versatile in its functionality.”
The ProSound F75 Ultrasound Processor offers a unique ergonomic capability with an adjustable height and depth of the keyboard and monitor that provides comfort to all endoscopists in a practice. The ProSound F75 has versatile capabilities for conducting quick tasks and examinations in every clinical setting. In addition, the device is forward and backward compatible which allows it to work with most endoscopic ultrasound (EUS) and endobronchial ultrasound (EBUS) scopes.
“We are very pleased to partner with Hitachi Aloka Medical, Ltd. and provide our customers with a complete solution for imaging that addresses both their clinical and operational needs,” said Luke Calcraft, President of the Medical Systems Group at Olympus Corporation of the Americas. “The ProSound F75 enables physicians to realize clinical efficacy through premier imaging and helps healthcare facilities to achieve efficient asset management through forward and backward scope compatibility of the platform.”
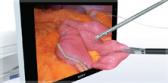
Olympus is a pioneer of endoscopic ultrasound (EUS) which combines ultrasound technology with endoscopy to better visualize the tissues of the digestive tract and adjacent anatomical structures inside the human body. Conventional ultrasound is performed by placing a transducer against the skin to produce images of internal organs. With EUS, the transducer is endoscopically inserted into the body via the digestive tract, putting it closer to the area of interest to obtain higher resolution images.
This technology addresses the key requirements of Healthcare Reform:
- Increased Quality of Care – EUS drives optimal patient outcomes through easy identification and more accurate staging of diseases and cancers in the GI tract.
- Decreased Costs – The forward and backward compatibility provides significant cost savings through lower cost of ownership and more efficient use of assets.
- Enhanced Patient Satisfaction – EUS provides a less invasive approach than conventional surgical diagnosis allowing physicians to view internal anatomy without making an incision.
According to the American Cancer Society, there are more than 125,000 newly diagnosed cases of gastrointestinal cancer each year in the United States with more than 91,000 deaths from these diseases in 2013. In addition, Truven Health estimates that the total number of EUS outpatient procedures will grow 15% from 2011 to 2016 and reach 235,000 procedures.
The ProSound F75 Ultrasound Processor will be showcased at the Digestive Disease Week (DDW) 2013 Annual Meeting to be held May 18-21 in Orlando, FL. Physicians are invited to visit booth #548 to further explore the benefits of the new ultrasound imaging platform.
For more information or to evaluate the ProSound F75 Ultrasound Processor, please contact your Olympus representative or call 800-848-9024.
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare.
For more information visit Olympus at www.olympusamerica.com.
Media Contact
Michael Levey, Olympus, 484-896-5120, michael.levey@olympus.com
About Hitachi Aloka Medical, Ltd. (Subsidiary of Hitachi Medical Corporation)
Hitachi Aloka Medical, Ltd. is committed to delivering advanced diagnostic ultrasound systems to meet the needs of physicians and patients. The company was formed when Aloka Co., Ltd. became a 100% subsidiary of Hitachi Medical Corporation on March 3, 2011 and changed its name to Hitachi Aloka Medical, Ltd. on April 1, 2011. Aloka commercialized medical ultrasound equipment for the first time in the world in 1960 and as Hitachi Aloka Medical, Ltd. continues to promote the ultrasound business under its highly valued Aloka brand. With its U.S. headquarters in Wallingford, CT, Hitachi Aloka Medical America employs local sales, service and clinical applications professionals across the nation. www.hitachi-aloka.com