Olympus Named Disruptive Innovator Award Finalist
ENDOCUFF to be Recognized at Inaugural Event on October 7 in Philadelphia
CENTER VALLEY, Pa., (September 17, 2018) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today that the ENDOCUFF® has been named a 2018 Disruptive Innovator Award Finalist in the Industry Break-through category by Healio Gastroenterology and Liver Disease Magazine. The inaugural awards program looks at the various changes in gastroenterology and how the Innovators have pushed the status quo toward the betterment of the field.
“Due to ENDOCUFF’s groundbreaking design and technology, physicians are achieving adenoma detection rate (ADR) improvements,” said Kurt Heine, Group Vice Present of the Endoscopy Division at Olympus America Inc. “There is no question that a device that helps avoid the suffering and costs of later stage cancer treatment is a disruptive innovation. We are honored to be included in this program as a finalist.”
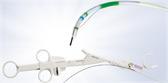
Placed on the distal tip of a colonoscope, ENDOCUFF’s unique hinged arms fall flat against the shaft of the scope to reduce slippage during forward advancement. During withdrawal, the arms flare out and stabilize the tip, gently stretching the mucosal surface to allow the physician thorough inspection of the anatomy during the search for polyps. The study results demonstrate that the design of ENDOCUFF is superior as a mucosal exposure device for colonoscopy.
Recently, a multi-center, randomized study on emerging colonoscopy technologies was conducted and published in Volume 88, No. 2 : 2018 of the prestigious GIE Journal of the American Society of Gastroenterologists. The study revealed that the use of ENDOCUFF produced gains in adenoma detection, even in the hands of physicians who are very skilled with standard instruments lacking adjunctive devices and with a high adenoma detection rate (ADR). ADR was 14% higher compared to HD colonoscopy without an adjunctive device.
ENDOCUFF is a 510(k) cleared, second generation, single-use device designed to give an optimal view of the entire colon throughout a colonoscopy procedure. As of 2016, the ENDOCUFF was discontinued and replaced with the next generation, ENDOCUFF VISION. Compared to the original ENDOCUFF, ENDOCUFF VISION has a single row of longer arms. These changes deliver yet more tip control throughout the colon without compromising intubation and improving loop management. Olympus Corporation of the Americas is the exclusive distributor of the device in the U.S. and Canada.
The Disruptive Innovators were nominated by Healio’s Editorial Board. Winners will be announced at the awards program on October 7, 2018 in Philadelphia.
# # #
About Olympus Medical Systems Group
Olympus is a global technology leader, crafting innovative optical and digital solutions in medical technologies; life sciences; industrial solutions; and cameras and audio products. Throughout our nearly 100-year history, Olympus has focused on being true to society and making people’s lives healthier, safer and more fulfilling.
Our Medical Business works with health care professionals to combine our innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing with their skills to deliver diagnostic, therapeutic and minimally invasive procedures to improve clinical outcomes, reduce overall costs and enhance quality of life for patients. For more information, visit medical.olympusamerica.com.